The FDA describes its requirements for human factors engineering in two documents:
- Applying Human Factors and Usability Engineering to Medical Devices (Feb 2016, final version)
- Content of Human Factors Information in Medical Device Submissions (Dec 2022, draft)
What the FDA calls “Human Factors Engineering” (HFE), IEC 62366-1 calls “Usability Engineering”.
The FDA defines the “Usability Engineering” as follows:
Application of knowledge about human behavior, abilities, limitations, and other characteristics to the design of medical devices (including software), systems and tasks to achieve adequate usability.
Scope
- Applying Human Factors and Usability Engineering to Medical Devices
- The purpose of the Human Factors Guidance is to recommend and guide manufacturers through human factors engineering processes during the development of new medical devices, focusing specifically on the user interface. That guidance provides relevant human factors definitions and recommends useful preliminary analysis and evaluation tools and validation testing that will enable manufacturers to assess and reduce risks associated with medical device use.
- Content of Human Factors Information in Medical Device Submissions
- Help manufacturers apply a risk-based approach when considering what human factors information to include in a marketing submission.
- It is intended to replace the FDA guidance “List of Highest Priority Devices for Human Factors Review”.
- It is intended to supplement, clarify and even replace Chapters 3 (Definitions), 9 (Documentation), and Annex A (HFE/UE Report) of the FDA Guidance “Applying Human Factors and Usability Engineering to Medical Devices” (HFE Guidance).
- The HFE Guidance defines the HFE process that results in the documents that must be submitted in whole or in part to the FDA. The second mentioned guidance document (Content of Human Factors) determines which documents have to be submitted.
Principles of Usability Engineering in Medical Device Design
- The ability of users to interact easily with the device, i.e., using the device should be as intuitive as possible
- The ability of users to interact and use the device error-free
- The device should work the way the clinician or user expects it to work
Three major elements of UE: User, User interface, Use Environment
The interaction of these 3 elements with device use result in either correct use or use error.
Human Factors Engineering: Which documents to submit
Determination of the category
The HF documentation depends on the risk of the device or the modification of an existing device:
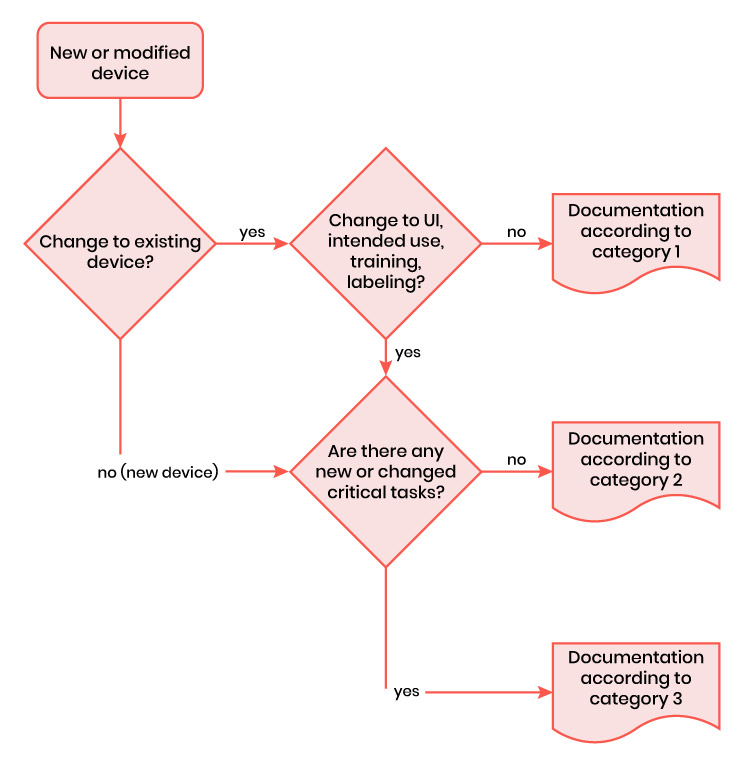 Determination of the documents to be submitted
Determination of the documents to be submitted
Documents
- Summary
- Description of intended use (incl. user, context of use, training)
- Description of the user interface
- Summary of known usage problems
- Summary of the preliminary evaluations
- Risk analysis (focus on usability) (incl. hazards, risks and description of critical tasks)
- Usability validation details
For Category 1, need to submit only summary document.
For Category 2, need to submit summary, Description of intended use, Description of the user interface, Summary of known usage problems
For Category 3, All documents need to be submitted from 1 to 7.
Human Factors Engineering: The HFE process
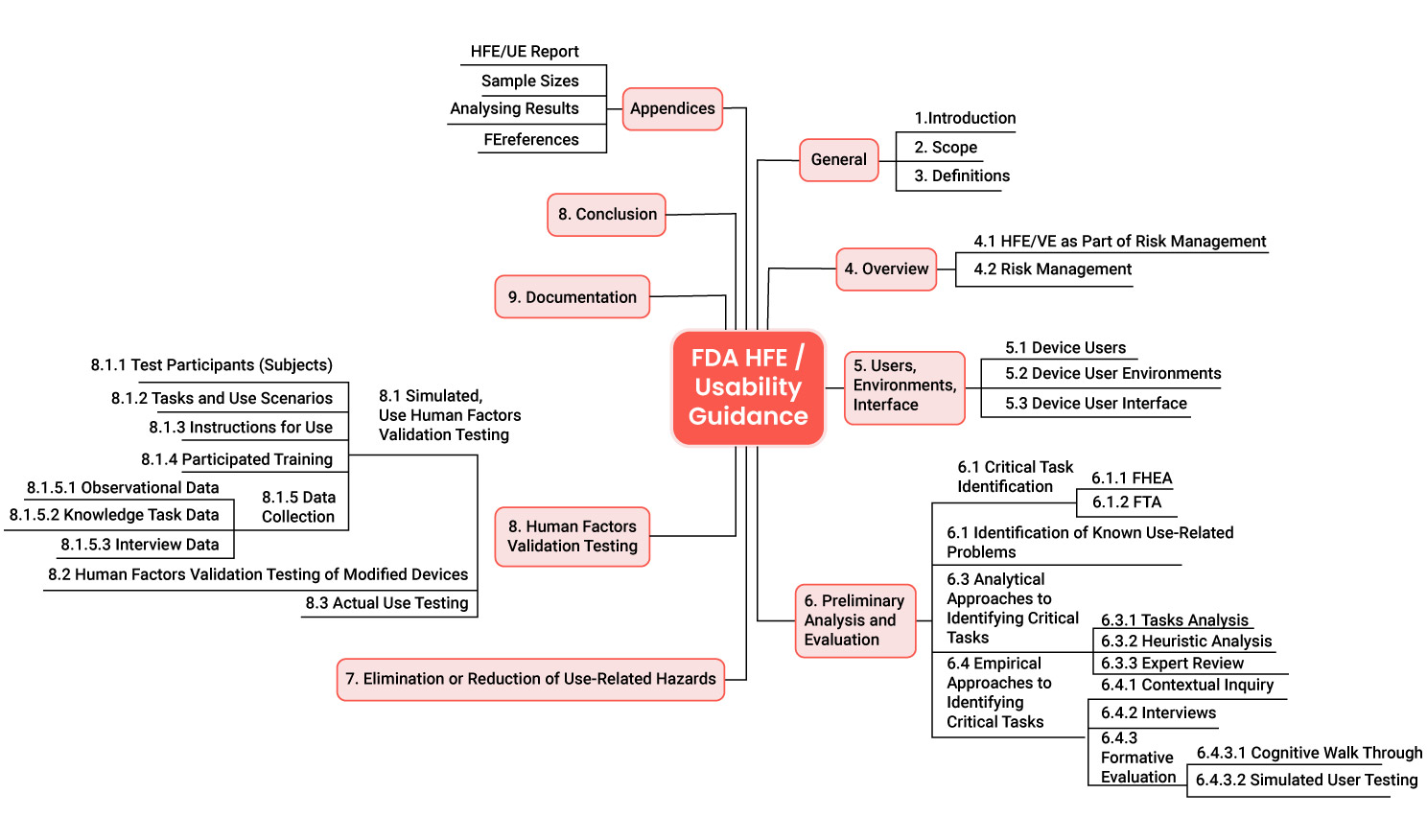
The guidance documents The FDA’s document “Applying Human Factors and Usability Engineering to Medical Devices” comprises 49 pages, ten chapters, and four appendices.
- This document contains definitions and an introduction to the subject in Chapters I to IV. The FDA describes concepts such as risk management, user, use environment, and user interface.
- It also describes the activities that manufacturers should go through as part of usability engineering:
- Risk analysis and identification of critical tasks (e.g., with FMEA, task analysis, analysis of known usage problems, heuristic evaluation, expert review)
- Formative evaluation (partly with comparable methods)
- Elimination of usage-related hazards
- HF Validation Testing
 Note: The requirements of IEC 62366-1 are very similar to the requirements in this guidance document, even if the terminology does not quite match. For example, the standard does not speak of Human Factors Validation Testing, but of Summative Evaluation.
Note: The requirements of IEC 62366-1 are very similar to the requirements in this guidance document, even if the terminology does not quite match. For example, the standard does not speak of Human Factors Validation Testing, but of Summative Evaluation.
In contrast to IEC 62366-1, the FDA hardly addresses the process. On the other hand, it describes the activities and methods in detail in the HFE Guidance. The latter are presented in a similar form in TR IEC 62366-2.
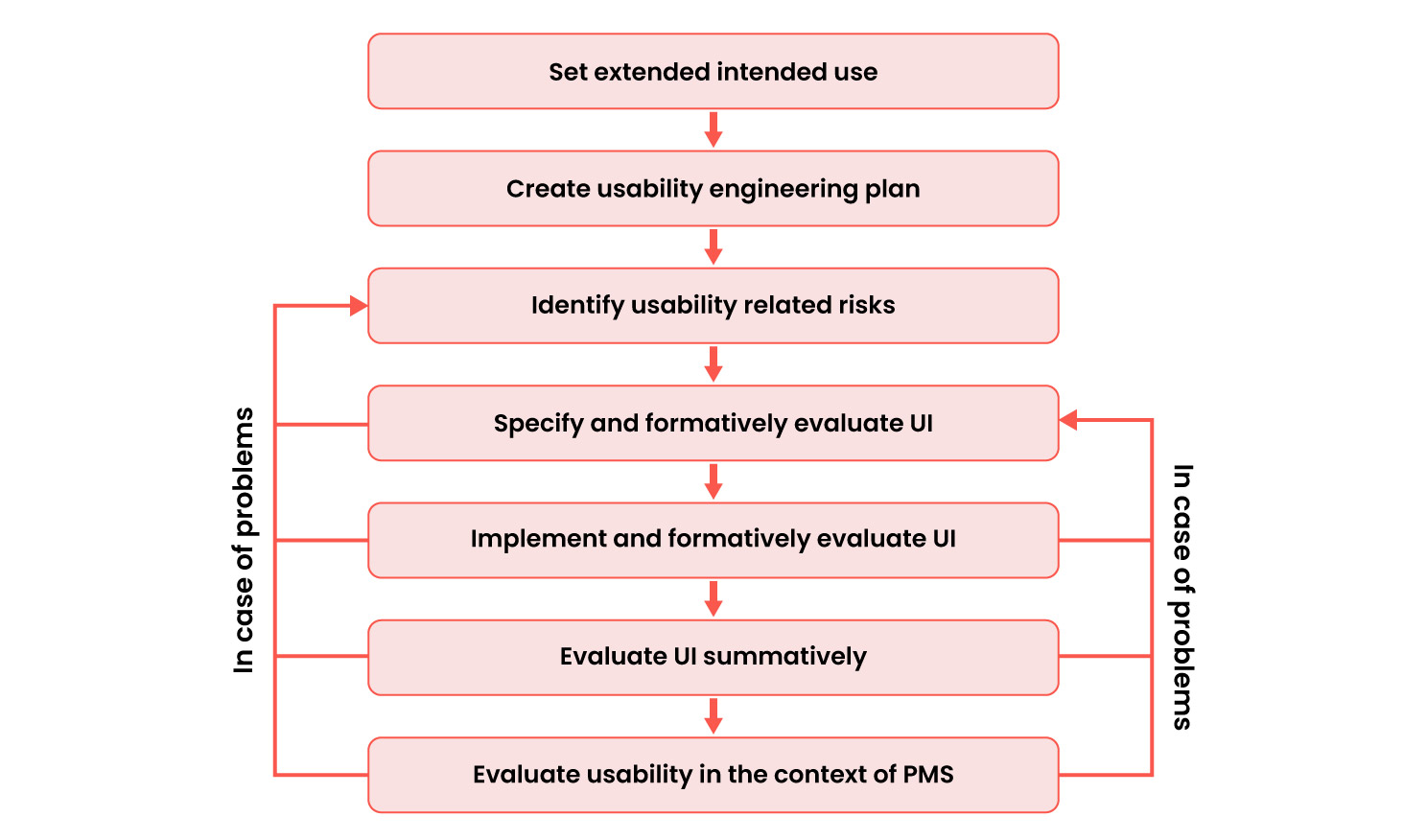
Implementation
A usability engineering process that meets both FDA and IEC 62366-1 requirements could be designed as follows:
 Conclusion
Conclusion
The FDA’s guidance documents on usability engineering are easy to understand and follow. The risk-based approach to compiling the documentation to be submitted helps to speed up approval procedures without endangering the safety of patients due to a lack of usability.
It is very useful to medical device manufacturers that IEC 62366-1 and the FDA’s HFE guidance are now highly aligned. This allows, for example, formative and summative evaluations to be conducted in a manner that meets the requirements of both legal areas.
For further information on Human Factors Engineering and Usability Guidelines for Medical Devices, please reach out to MakroCare today!


